Essential Elements of Hazardous Drug Compounding | On-demand version
Please note that this is the on-demand version of the course - the live event was recorded on October 16-17, 2021. The description below reflects the live event; as such, some statements may not apply.
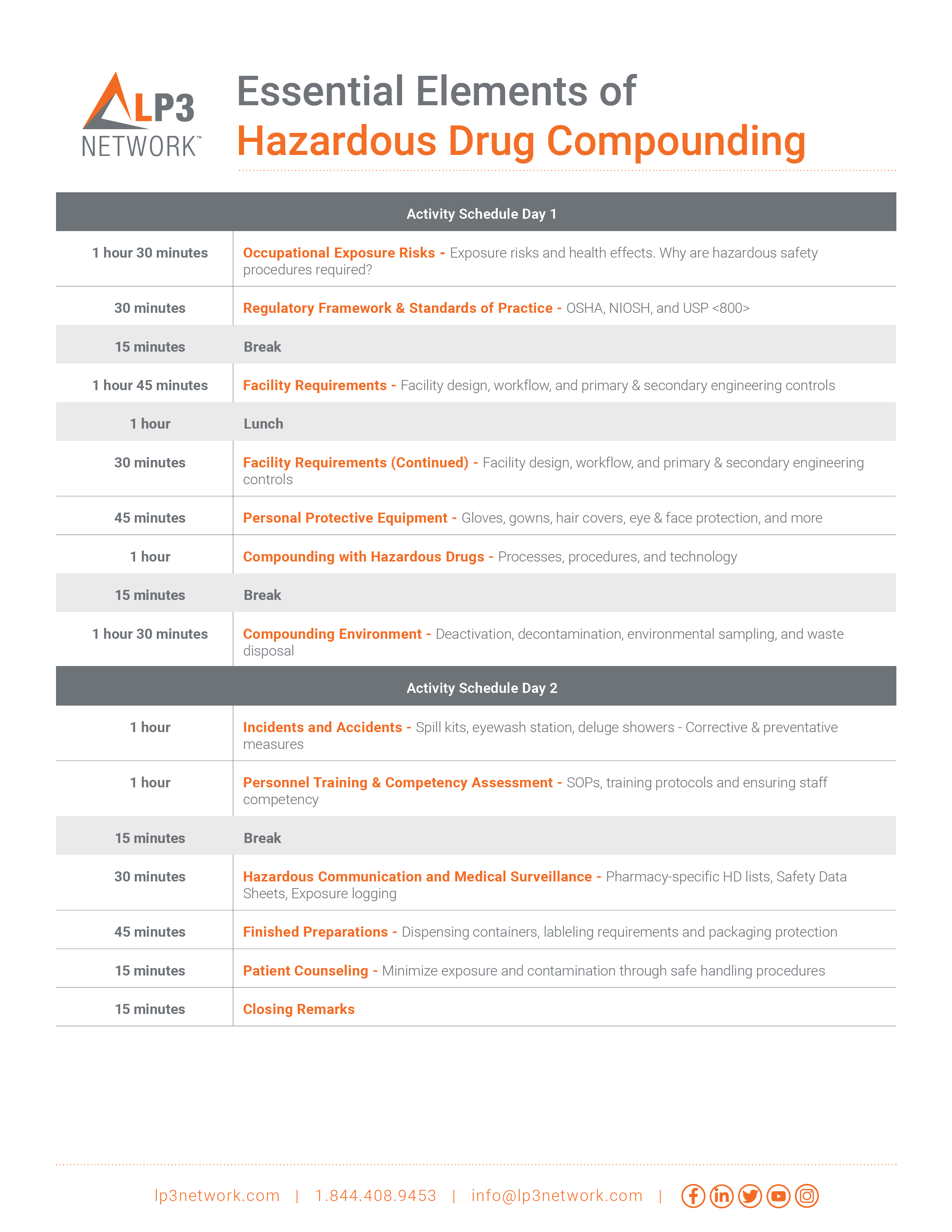
Course Format
1.5-day live virtual seminar (12 hours) that allows you to connect and interact with peers and the facilitator online, in real time, through open mic Q&A sessions, group discussions, polls, and online chats.
Course Description
Are you currently compounding with cyclosporine, estradiol, fluorouracil, phenytoin, progesterone, spironolactone, tacrolimus, testosterone, or any other drug found on the NIOSH List of Antineoplastic and Other Hazardous Drugs? If yes, then you want to comply with USP General Chapter <800> Hazardous Drugs – Handling in Healthcare Settings.
This application-based program is designed to take factual information presented in published USP, OSHA, NIOSH, and other healthcare guidelines and provide practical and applicable solutions to achieving compliance and safety within your practice. This program takes abstract concepts and makes them tangible and sensible. Participants will not just learn about the theory of facility requirements, they will develop their own facility blueprints and discuss and optimize them as a group. Participants will learn how to assess risk from personnel training & competency, to personal protective equipment (PPE), to drug handling, compounding processes, cleaning & deactivation, use of closed-system transfer devices, engineering controls, storage, and more. Participants will go through applicable scenarios and real-life situations that will shed light on exposure risk and how to correct it. The practical application and implementation of the requirements outlined in USP <800> can be challenging. This training provides you with the resources and insight for transitioning into compliance or maintaining compliance with USP <800>.

Concerned about USP <800> and the implications for you and your practice? Browse our FAQ for an idea of the topics we address in full during our Hazardous Drug Compounding seminar.
Intended audience
Pharmacists, pharmacy technicians, pharmacy managers, quality assurance officers, and compliance specialists looking for practical and applicable solutions to achieve compliance with USP General Chapter <800> Hazardous Drugs – Handling in Healthcare Settings.
Learning Objectives
FOR PHARMACISTS:
- Describe the hazardous compounding regulatory framework and standards of practice, including OSHA, NIOSH and USP <800>.
- Examine the risks of working with hazardous drugs in the pharmacy and healthcare continuum.
- Construct a pharmacy-specific hazardous drug list by assessing the occupational risks associated with hazardous drugs.
- Evaluate the critical strategies required to protect personnel, patients, and the environment when handling hazardous drugs in a healthcare setting.
- Assess hazard communication and training requirements.
- Contrast containment primary engineering controls (C-PECs) and their application.
- Interpret facility design plans for non-sterile and sterile hazardous drug compounding.
- Manage safe handling practices required for hazardous drug handling and compounding.
- Evaluate deactivation and decontamination procedures.
- Select personal protective equipment based on the NIOSH tiered approach.
- Demonstrate donning and doffing procedures.
- Examine the advantages of medical surveillance and environmental monitoring.
FOR TECHNICIANS:
- Describe the hazardous compounding regulatory framework and standards of practice, including OSHA, NIOSH and USP <800>.
- List the risks of working with hazardous drugs in the pharmacy and healthcare continuum.
- Construct a pharmacy-specific hazardous drug list by assessing the occupational risks associated with hazardous drugs.
- Outline strategies required to protect personnel, patients, and the environment when handling hazardous drugs in a healthcare setting.
- Recognize the importance of hazard communication and training requirements.
- Describe the different containment primary engineering controls (C-PECs) and their rationale for use.
- Reproduce facility design plans for non-sterile and sterile hazardous drug compounding.
- Outline safe handling practices required for hazardous drug handling and compounding.
- Describe deactivation and decontamination procedures.
- Select personal protective equipment based on the NIOSH tiered approach.
- Demonstrate donning and doffing procedures.
- List the advantages of medical surveillance and environmental monitoring.
FINANCIAL SUPPORT
This learning activity has received financial support from MEDISCA Inc. in the form of an educational grant.
COPYRIGHT
The live activity workbook is copyright © 2016-2021 LP3 Network.
Hardware/software requirements
| Web browser | Google Chrome (most recent 2 versions) Mozilla Firefox (most recent 2 versions) Internet Explorer v11 Apple Safari (most recent 2 versions) Microsoft Edge (most recent 2 versions) |
Internet connection | Computer: 1 Mbps or better (broadband recommended) Mobile device: 3G or better (WiFi recommended) |
Software | No additional software needed |
Hardware | 2GB of RAM (minimum), 4GB or more of RAM (recommended) |
Testimonials
"The instructor has a lot of experience and is very enthusiastic, making the course very enjoyable. I definitely recommend this seminar to my peers. It taught me the skills to develop my practice and I am already applying what I learned!”
Ana Gonzalez | Pharmacist
FINANCIAL SUPPORT:
This learning activity has received financial support from MEDISCA Inc. in the form of an educational grant.
COPYRIGHT:
This CE Activity is Copyright © 2016-2026 LP3 Network.
CHRISTINE ROUSSEL, PharmD, BCOP, BCSCP |  |
Dr. Christine Roussel is a Board Certified Oncology Pharmacist Board Certified Sterile Compounding Pharmacist with a bachelor's in Toxicology and doctorate in Pharmacy from Philadelphia College of Pharmacy. She is the Senior Executive Director of Pharmacy, Laboratory and Medical Research Doylestown Hospital (Pennsylvania), Adjunct Assistant Professor at USciences, Facilitator for LP3 Network, and consultant for MEDISCA Network. On top of her day job of overseeing the clinical and operational aspects of patient care, she provides over 100 hours of education to pharmacists, pharmacy technicians, physicians, and nurses each year.
Dr. Roussel focuses on occupational exposure to hazardous drugs, sterile/non-sterile compounding, clinical and legislative topics, such as medical cannabis, opioid stewardship, drug diversion prevention, pharmaceutical waste and contamination. She is a consultant for cleanroom facility design. She has authored articles on occupational exposure to hazardous drugs, as protecting healthcare providers’ DNA is her primary passion! Her most recent publication is: Meta-analysis of chromosomal aberrations as a biomarker of exposure in healthcare workers occupationally exposed to antineoplastic drugs. In 2018 she was nationally recognized as “40 Under 40 in Oncology”
She is active in many professional organizations, including Delegate for the American Society of Health Systems Pharmacy, President of the Pennsylvania Society of Health Systems Pharmacy, Board of Directors for International Society of Cannabis Pharmacists, American Industrial Hygiene Association, and the Association for Compounding Pharmacists (ACP).
Editor
 ADAM BEACH, PhD ADAM BEACH, PhDLearning Technology Manager Disclosure: None |  |
This recorded seminar is accredited for Pharmacists and Pharmacy Technicians by the Accreditation Council for Pharmacy Education (ACPE) through CPE Consultants, LLC.
Total CPE Credits: 12 CPE Hours = 1.2 CEUs
This activity is a recording from a live virtual seminar and those that claim credit for the live virtual seminar should not claim credit for this activity.
| ON-DEMAND RECORDING | |
| TYPE | Application-based |
| UAN | 0864-9999-21-055-H07-P/T |
| CREDITS | 12 CPE Hours = 1.2 CEUs |
| RELEASE DATE | October 16, 2021 |
| EXPIRATION DATE | October 16, 2024 |
Completion Requirements
- View the on-demand recording of the seminar, which will only be available for 30 days following your enrollment.
- Complete a learning assessment (25 questions) with a passing grade of 70%.
- Submit a completed activity evaluation form online.
*Important Note: Steps 2 and 3 (the on-demand learning assessment and on-demand evaluation) must be completed within 6 weeks of enrollment. After this time, you will no longer be able to claim credits for this activity.
When the aforementioned steps have been completed and approved:
- Pharmacists and Pharmacy Technicians registered in the United States will obtain a statement of completed credits on their NABP e-Profile within 60 days.
- Pharmacists and Pharmacy Technicians registered elsewhere will be able to download a statement of participation within 60 days.
- All participants will receive an automated email from LP3 Network advising participants that they can download the LP3 Network certificate.
 | CPE Consultants, LLC is accredited by the Accreditation Council for Pharmacy Education as a provider of continuing pharmacy education. |
International participants should verify with their respective governing board for accreditation equivalency.

 Facebook
Facebook X
X LinkedIn
LinkedIn Forward
Forward