Aventura, FL – October 22-23, 2016
This practice-based activity is divided into four sections: modern facility design concepts for non-hazardous and hazardous compounding, establishing a strategic collaborative practice model, working with advanced drug delivery systems, and quality controlled compounding.
The activity begins by discussing modern facility design concepts in adherence with current standards of practice. It explores advantages and disadvantages related to the design of compounding facilities for non-hazardous and hazardous drug compounding and explores the many variables that impact facility design, including functional and operational parameters of secondary engineering controls (SECs), containment-secondary engineering controls (C-SECs), primary engineering controls (PECs), and containment-primary engineering controls (C-PECs). Furthermore, factors that impact functional and operational performance measures will be used to establish design strategies for an efficient and effective facility design. Participants will be given the opportunity to complete a questionnaire that helps summarize the parameters of a compounding practice, in order to define their own facility requirements. Next, participants will engage in designing an advanced facility workflow schematic for a non-hazardous and hazardous practice. Additional factors that will be taken into account include positive and negative pressure, air pressure differentials, air changes per hour (ACPH), air velocity, air reuptake, and HEPA filter coverage requirements for air exhaust systems.
The focus then shifts to challenging the compounding pharmacist to examine a clinically-based, collaborative strategy that will redefine the pharmacist, physician and patient relationship. Participants will explore niche market-specific, collaborative clinical assessment profile variables in an effort to increase communication and interaction with prescribers. This will help solidify relations, build confidence with prescribers, build an evidence-based database in support of the compounding practice, and bring a clinical superiority to the patient care challenge.
Next, drug delivery systems will be reviewed and innovative research outlining novel concepts in bioavailability optimization for poorly absorbed (large and/or lipophilic) drugs is presented; delivery systems for oral, rectal, and topical dosage forms are discussed. This can provide the compounding pharmacist with a clear advantage in the marketplace. A comprehensive set of factors that impact bioavailability is examined in detail. Each route of delivery will then be further examined, focusing on topical, oral, and rectal routes. Relationships are defined and established between drug agent, barrier to absorption, and base/delivery system. The criteria for utilization and scientific are outlined followed by the demonstration of advanced delivery systems. Each route of delivery is then assessed for its respective factors that impede absorption and the corresponding solution to the obstacle presented.
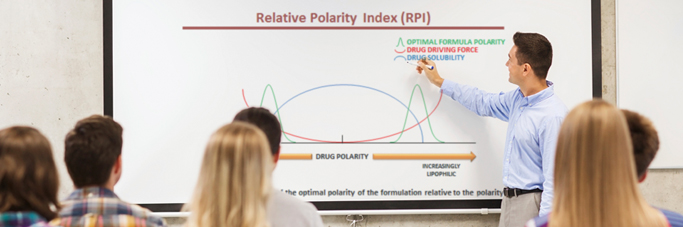
Datasets from quantitative structural activity relationships (QSARs) are used to identify functional characteristics of drug agents. The n-Octanol-water partition coefficient, permeability coefficient, diffusion coefficient, and distribution coefficient each reveal unique characteristics or potential activity of drug agents across barriers to absorption. Details are provided on Hydrophilic-lipophilic balance (HLB) and pH manipulation in order to increase permeation. Permeation-enhancing agents, surfactants, and emulsifying agents are brought together to form the basis of the advanced delivery system. The interrelationship between these agents, held together at specific concentrations, results in a relative polarity index conducive to enhanced permeation. The delivery system utilizes and interacts with physical structures within the barrier to absorption to create an opportunity for improvement in permeability, without deleterious side effects to the barrier itself. The result is a potential for improvement in clinical outcome, potential for reduction in drug concentration requirements in the formulation, and a potential for reduction in deleterious side effects to the patient.
The next topic emphasizes an all-important aspect of a compounding practice, Quality Controlled Compounding (QCC). QCC can be divided into three sub-categories: efficacy, reliability, and validity. These three sub-categories are applied to specific pharmaceutical calculations, personnel performance assessment, and process verification, all of which increase the quality assurance and quality control of finished, compounded preparations. Each of the three sub-categories will be further subdivided, allowing the participant to apply a broad range of analytical parameters to the developmental process for a master formulation record, which includes our newly-defined, advanced drug delivery systems.
Intended audience
Learning Objectives
FOR PHARMACISTS:
- Propose a non-sterile compounding facility that takes into account primary and secondary engineering controls for the following parameters of practice: non-hazardous drug, hazardous drug, and advanced delivery system compounding.
- Assess a non-sterile compounding practice and its day-to-day operation through the use of a questionnaire to assist in determining facility design requirements.
- Defend proposed functional and operational parameters of a non-sterile compounding facility for its primary and secondary engineering control selection, assigned positive and negative pressure with related pressure differentials, suggested HEPA filter coverage for air exhaust system requirements, and resultant air changes per hour (ACPH), air velocity and air reuptake.
- Assess a clinically-based collaborative strategy designed to help solidify relations, build confidence with prescribers, build an evidence-based database in support of the compounding practice, and bring a clinical superiority to the patient care challenge.
- Assess novel concepts in bioavailability optimization for poorly absorbed (large and/or lipophilic) drugs; applies to oral, rectal and topical dosage forms.
- Assess specific characteristics of quantitative structural activity relationships used to identify functional characteristics of drug agents.
- Defend the use and application of: a quantitative, structural activity relationship; n-Octanol-Water partition coefficient; permeability coefficient; diffusion coefficient; distribution coefficient; as well as the manipulation of the hydrophilic-lipophilic balance and pH of a compounded mixture, in support of proposed bioavailability optimization techniques.
- Value a set of criteria intended to support the compounding pharmacist in the decision-making process to utilize an advanced drug delivery system.
- Formulate sound clinical judgement before suggesting/utilizing an advanced drug delivery system in the context of individualized patient care.
- Select and apply quality control compounding parameters to ensure the efficacy, reliability and validity of finished preparations.
- Support the use of unit metered-dose calculation methods to ensure drug concentrations are within acceptable margins of error by employing specific pharmaceutical calculations.
- Compose methods to ensure optimized personnel performance by employing process verification strategies.
- Compose process development strategies used in the detailing of a master formulation record.
An unrestricted educational grant has been provided by MEDISCA Inc.
The preferred hotel accommodation is the Residence Inn by Marriott Miami Aventura Mall
For reservations, please call 1-786-528-1001 or click here.

Based on availability. Payment for hotel accommodations is at the expense of the activity participant.

MARK FILOSI, BS Pharm, RPh
Compounding Pharmacist and Co-Founder, Family Care Pharmacy
Disclosure: Accreditation Commission for Health Care Surveyor; MEDISCA Consultant
Mr. Filosi is a compounding pharmacist for a thriving non-sterile and sterile practice with over 20 years of experience. He is also a surveyor for the Pharmacy Compounding Accreditation Board (PCAB), a service of the Accreditation Commission for Health Care (ACHC). Additionally, Mr. Filosi is the owner of Family Care Pharmacy in Plant City, Florida, where he is responsible for the sales and marketing of the compounding segment of his business. His compounding practice ranges from non-sterile hormone preparations to high-risk intrathecal preparations.
He graduated cum laude from Fitchburg State College in Fitchburg, Massachusetts, and then went on to graduate cum laude in Pharmacy from Massachusetts College of Pharmacy in Boston, Massachusetts. Today, Mr. Filosi is a preceptor for Florida A & M University, University of Florida and Massachusetts College of Pharmacy.
CPE Credits: 16 CPE hours = 1.6 CEUs
Joint Accreditation Status (University of Florida College of Pharmacy / LP3 Network)
Activity type: Practice-based
UAN: 0012-9999-16-004-L04-P
To receive CPE credits for the live component, participants must demonstrate full and satisfactory participation, and submit a completed evaluation to the University of Florida College of Pharmacy.
Participants registered in the United States can obtain a statement of credit from their NABP e-profile. The University of Florida College of Pharmacy will report CPE credits to the CPE Monitor. Participants registered other than in the United States will receive a statement of credit by mail.

International participants should verify with their respective governing board for accreditation equivalency.
Price
A written request must be sent to LP3 Network requesting “Cancellation without Transfer” to an alternate live activity date. If cancellation occurs at greater than or equal to 31 days from the live activity date, then the registrant will receive a 75% refund; 15-30 days a 50% refund; and less than or equal to 14 days no refund.
TRANSFER POLICY:
The transfer policy is only in effect if requested greater than or equal to 14 days prior to the original live activity date. Failure to submit the “Transfer Request” at least 14 days prior to the original live activity date will default to the “Cancellation and Refund Policy”. The transfer policy can only be applied one (1) time. Transfer to a new live activity date must be within the current calendar year. Failure to attend the new live activity will result in no refund and will no longer be transferable. Confirmation of the new activity date is subject to approval by LP3 Network based on the number of available seats within the selected live activity. There are no refunds, returns, or transfers upon purchase of the home study.
CURRENT PROMOTION:
20% Off
 Use coupon code 20LP316 upon checkout to receive 20% off this educational training.
Use coupon code 20LP316 upon checkout to receive 20% off this educational training.
PURCHASE INFORMATION:
Don’t see the Add to Cart button? Please try to Log In, Create an Account or Contact Us for help!

 Facebook
Facebook X
X LinkedIn
LinkedIn Forward
Forward