Los Angeles, CA – August 30-September 1, 2024 | Achieving Best Practices: Sterile Compounding
Achieving Best Practices: Sterile Compounding

This program is a PTCB-recognized sterile compounding education / training program for those who are pursuing the PTCB Certified Compounded Sterile Preparation Technician® (CSPT®) designation. This program has recently been updated to align with the new USP <797> chapter to go into effect on November 1, 2023, and is the sterile training of choice to meet the designated person and pharmacy personnel training requirements.
This event will take place in the local time, Pacific Daylight Time (PDT)
Course Format
Home Study (30 hours) with learning assessment that reviews fundamental principles of sterile compounding in preparation for the live event.
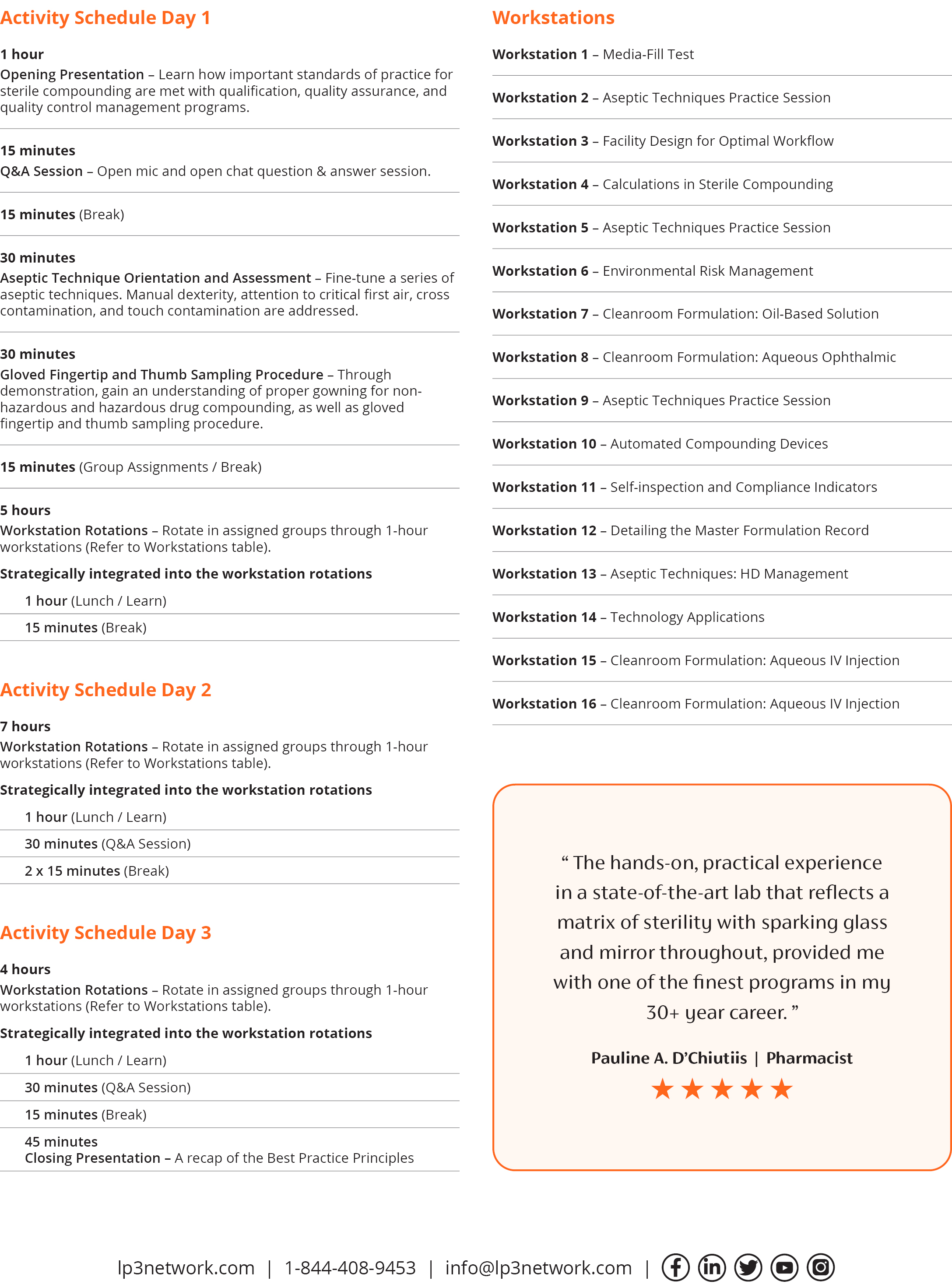
Live Training (20 hours in 2.5 days) consisting of sixteen 1-hour rotating small-group workstations where you will practice and be assessed for competency on various functions essential for sterile compounding.
Course Description
The Home Study component of this activity addresses the fundamentals of quality management, calculations, and principles & techniques surrounding non-hazardous and hazardous compounded sterile preparations. To review the full activity description for the Home Study click here.
The Live Training component transforms theory into practice, bringing standards of practice to life. Setting the stage, an opening presentation brings to the forefront key elements of qualification, quality assurance, and quality control. Following this, over 2.5 days, participants will rotate through a series of sixteen 1-hour workstations as they rehearse the role of compounder, designated person, inspector, and cleanroom designer. Participants will perform media-fill tests, practice aseptic techniques for non-hazardous and hazardous drug compounding, design facilities for optimal workflow, perform calculations for sterile compounding, work with automated compounding devices, detail master formulation records, self-inspect, apply different technologies, and prepare 4 sterile medications in a state-of-the-art ISO certified three-room cleanroom suite.
Intended audience
Pharmacists, pharmacy technicians, pharmacy managers, and quality assurance & control officers looking for hands-on, practical training in sterile compounding.
Learning Objectives
PHARMACISTS
- Demonstrate donning and doffing, hand hygiene, aseptic manipulations and sanitization techniques
- Demonstrate the use of closed system transfer devices for hazardous drug compounding
- Prepare compounded sterile preparations in an ISO classified three-room cleanroom suite
- Assess and perform adjustment calculations for drug form and certificate of analysis-related indications
- Perform overage calculations for a compounded sterile preparation
- Sketch a cleanroom suite for optimal workflow
- Complete a Master Formulation Record
- Explain how to perform the gloved fingertip and thumb sampling and a media-fill test
- Summarize findings of a risk assessment, and then construct a corrective action and preventive action plan
- Summarize findings following an inspection, and then construct a corrective action and preventive action plan
- Operate electromechanical equipment (e.g., balance, automated compounded device, particulate samplers (non-viable and viable), incubator, sterilizers (steam and dry heat), biological indicator and chemical integrator and pH meter)
PHARMACY TECHNICIANS
- Demonstrate donning and doffing, hand hygiene, aseptic manipulations and sanitization techniques
- Demonstrate the use of closed system transfer devices for hazardous drug compounding
- Prepare compounded sterile preparations in an ISO classified three-room cleanroom suite
- Solve adjustment calculations for drug form and certificate of analysis-related indications
- Solve overage calculations for processing, sterility and endotoxin testing
- Sketch a cleanroom suite for optimal workflow
- Complete a Master Formulation Record
- Explain how to perform the gloved fingertip and thumb sampling and a media-fill test
- Describe findings of a risk assessment, and then construct a corrective action and preventive action plan
- Describe findings following an inspection, and then construct a corrective action and preventive action plan
- Operate electromechanical equipment (e.g., balance, automated compounded device, particulate samplers (non-viable and viable), incubator, sterilizers (steam and dry heat), biological indicator and chemical integrator, pH meter, and more)
Testimonials
“Having recently taken a position in a hospital setting, I decided to build on my basic knowledge of sterile compounding. I chose LP3 Network again, in my opinion, they are recognized as experts in this facet of pharmacy, and justifiably so. I highly recommend this program to any person who has the desire to excel in their compounding skills, because you can only be one of the best, if you learn from the best.”
“The course provided me with valuable, relevant and applicable information on sterile compounding in the comfort of a beautiful practice site. The combination of highly knowledgeable instructors, didactic written materials, hands-on-practical experience in a state-of–the-art lab that reflects a matrix of sterility with sparkling glass and mirrors throughout, provided me with one of the finest programs in my 30+ year career. I came away with having a proactive, rather than reactive approach to the preparation of compounded medications. My confidence has been 'compounded', no pun intended!
Pauline A. D’Chiutiis | Pharmacist
“What a wonderful program! The instructors use different types of learning throughout the entire program to grab the attention of every type of learner, through lecture, hands-on, and peer education. The practical component puts all the literature into perspective and the instructors teaching this course are absolutely A1!
Stephanie Armstrong | Pharmacy Assistant
FINANCIAL SUPPORT
This learning activity has received financial support from MEDISCA in the form of an educational grant.
COPYRIGHT
This CE Activity is Copyright © 2006-2026 LP3 Network.
Travel information
Please proceed to Room G32 (Located on the basement level of the building) for sign-in and registration; the Lab Room is G24/G25.

The preferred hotel accommodation is the The Wayfarer Downtown LA, Tapestry Collection by Hilton
For online reservations, please click here or call +1-213-285-4400
Based on Availability. Payment for hotel accommodations is at the expense of the activity participant.

Facilitators will be announced closer to the activity start date – please check back soon!
Note: Facilitators are subject to change.
Editor
| NEIL COHEN, BSc CE Program Developer Disclosure: None |  |
Contributors
Pharmacists & Pharmacy Technicians
Both the Home Study and Live Event are accredited for Pharmacists and Pharmacy Technicians by the Accreditation Council for Pharmacy Education (ACPE) through CPE Consultants, LLC.
Total CPE Credits: 50 CPE Hours = 5.0 CEUs
| Home Study | Live Event | |
| Type | Knowledge-based | Application-based |
| UAN | 0864-9999-23-001-H07-P/T | 0864-9999-23-006-L07-P/T |
| Credits | 30 CPE hours = 3.0 CEUs | 20 CPE hours = 2.0 CEUs |
| Release Date | January 1, 2023 | January 16, 2023 |
| Expiration Date | January 1, 2026 | January 16, 2026 |
Pharmacy Technicians
This program is a PTCB-recognized sterile compounding education / training program for those who are pursuing the PTCB Certified Compounded Sterile Preparation Technician® (CSPT®) designation.
Completion Requirements
- Complete home study learning assessment with a score of 70% and submit a completed home study evaluation.
- Attend the lab training in full.
- Submit a completed live training evaluation form online within 14 days.
When the aforementioned steps have been completed and approved:
- Pharmacists and Pharmacy Technicians registered in the United States will obtain a statement of completed credits on their NABP e-Profile within 60 days.
- Pharmacists and Pharmacy Technicians registered elsewhere will receive a statement of completion by email within 60 days.
- All participants will receive an automated email from LP3 Network advising participants that they can download the LP3 Network certificate.
 | CPE Consultants, LLC is accredited by the Accreditation Council for Pharmacy Education as a provider of continuing pharmacy education. |
International participants should verify with their respective governing board for accreditation equivalency.
CANCELLATION AND REFUND POLICY
In the event that LP3 Network cancels a course, LP3 Network's sole liability shall be course repayment. In no event shall LP3 Network be responsible for any costs related to travel and/or accommodation. A written request must be sent to LP3 Network Inc. requesting ‘Cancellation without Transfer’ to an alternate live activity event date. If cancellation occurs at greater than or equal to 31 days from live activity event date, then registrant will receive a 75% refund; 15-30 days a 50% refund; and less than or equal to 14 days no refund. There are no refunds, returns, or transfer requests upon purchase of a home study, digital work book, webinar, and eLearning modules. LP3 NETWORK INC. shall be excused from any delay caused by reason of any occurrence or contingency beyond its reasonable control (a “Force Majeure”), including but not limited to, acts of God, hurricane, earthquake, labour disputes, strikes, riots, war, and governmental requirements. The obligation to pay money to LP3 NETWORK INC. in a timely manner is absolute and shall not be subject to this Force Majeure provision. In such event, LP3 NETWORK INC. will not issue any refunds, only transfer to an alternate live activity event date.
TRANSFER POLICY
The transfer policy is only in effect if requested greater than or equal to 14 days prior to the original live activity date. Failure to submit the “Transfer Request” at least 14 days prior to the original live activity date will default to the “Cancellation and Refund Policy”. The transfer policy can only be applied one (1) time. Transfer to a new live activity date must be within the current calendar year. Failure to attend the new live activity will result in no refund and will no longer be transferable. Confirmation of the new activity date is subject to approval by LP3 Network based on the number of available seats within the selected live activity. There are no refunds, returns, or transfers upon purchase of the home study.
Home Study Version
Please note: Canadian participants will automatically receive access to the Canadian version of the Home Study. All other participants will automatically receive access to the US version of the Home Study. If you would like to switch versions, please contact us.

 Facebook
Facebook X
X LinkedIn
LinkedIn Forward
Forward

