Sterile Compounding: Certificate Program | Canadian Version

FORMAT
The Sterile Compounding: Certificate Program is a 2.5 day in-person program, comprised of two components:
- Sterile Compounding: Home Study Manual - must be completed prior to the hands-on lab training.
- 8 chapters | 30 contact hours | 3.0 CEUs.
- Sterile Compounding: Hands-on Lab Training.
- 2.5 days | 20 contact hours | 2.0 CEUs.
- Value-added material included - Sterile Compounding: Labs Online. This material is supplemental to the hands-on lab training and is not part of the requirements to complete the Certificate Program.
- 25+ videos | 3 contact hours.
Intended audience
- Designated Persons/Compounding Supervisors
- Personnel with oversight responsibilities
- Compounding Personnel (Pharmacists and Pharmacy Technicians)
- Cleaning and disinfection personnel
- Inventory control managers
- Personnel specialized in allergenic extracts
- Inspectors
DESCRIPTION
Home Study
The Sterile Compounding: Home Study Manual component of this activity addresses the fundamentals of quality management, calculations, and principles & techniques surrounding non-hazardous and hazardous compounded sterile preparations.
To review the full activity description for the Sterile Compounding: Home Study Manual click here.
Lab Training
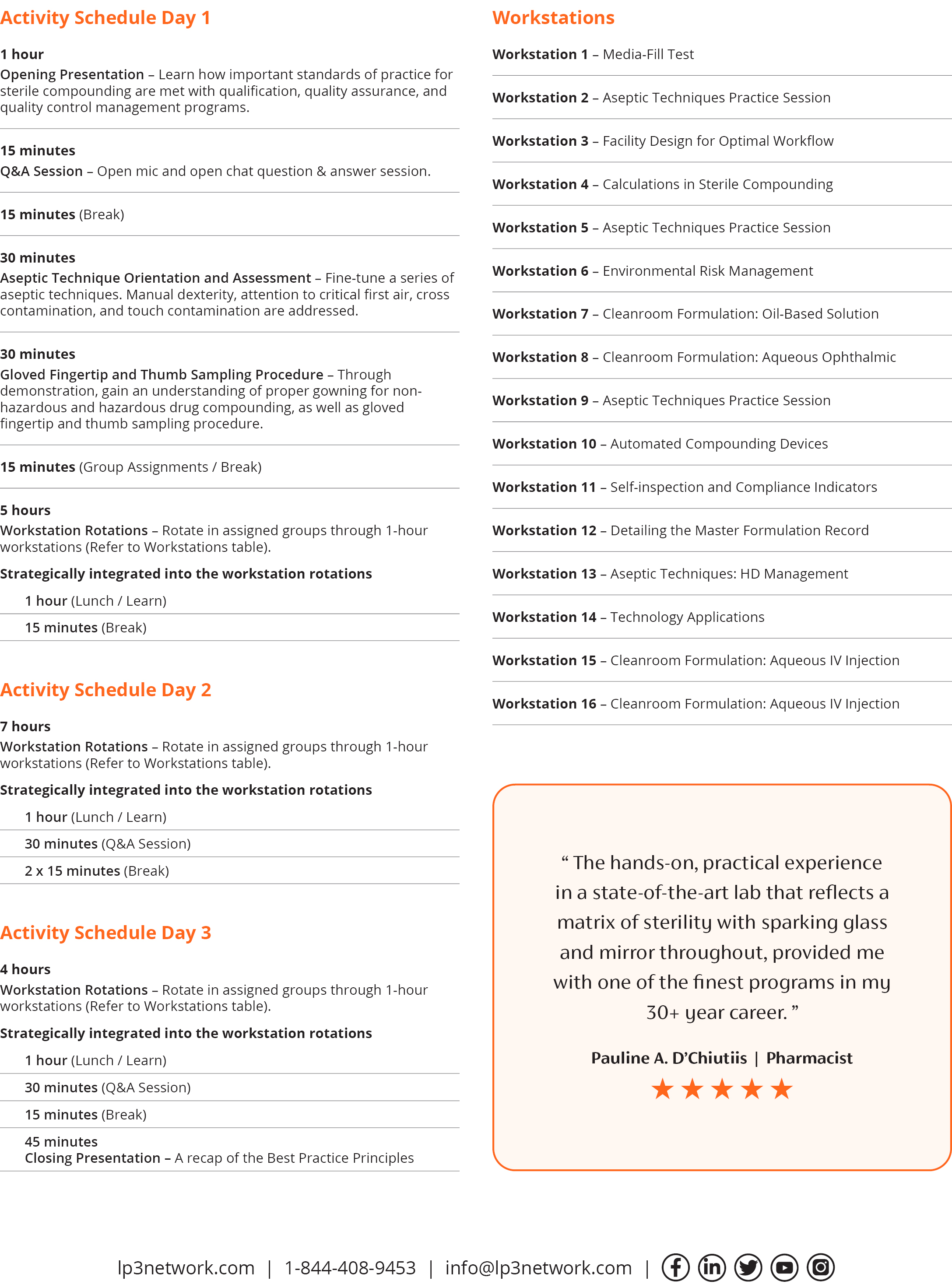
This Sterile Compounding: Hands-on Lab Training is an effective training method for novice-to-experienced sterile compounding personnel and transforms theory into practice, bringing standards of practice to life.
Participants will rotate through a series of sixteen 1-hour workstations as they rehearse the role of designated person, inspector, cleanroom designer, and most importantly sterile compounder. Participants will focus on aseptic technique and aseptic processing for non-hazardous and hazardous drug compounding, explore facility design for optimal workflow, experience methods in environmental risk management, scale up with automated compounding devices, fine tune calculations for sterile compounding, detail a master formulation record and prepare four sterile medications in a state-of-the-art three-room cleanroom suite.
Sterile Compounding: Labs Online
Sterile Compounding: Labs Online is a convenient, self-paced, audiovisual experience for your sterile compounding needs, complementing the Sterile Compounding: Hands-on Lab Training. With over 25 on-demand videos and over two hours of video recordings, Labs On-Demand for sterile compounding features a face-to-face dialogue with an experienced sterile compounding pharmacist on garbing of non-hazardous and hazardous personal protective equipment, cleanroom workflow, and aseptic techniques.
Learning Objectives
Pharmacists
- Demonstrate donning and doffing, hand hygiene, aseptic manipulations and sanitization techniques
- Demonstrate the use of closed system transfer devices for hazardous drug compounding
- Prepare compounded sterile preparations in an ISO classified three-room cleanroom suite
- Assess and perform adjustment calculations for drug form and certificate of analysis-related indications
- Perform overage calculations for a compounded sterile preparation
- Sketch a cleanroom suite for optimal workflow
- Complete a Master Formulation Record
- Explain how to perform the gloved fingertip and thumb sampling and a media-fill test
- Summarize findings of a risk assessment, and then construct a corrective action and preventive action plan
- Summarize findings following an inspection, and then construct a corrective action and preventive action plan
- Operate electromechanical equipment (e.g., balance, automated compounded device, particulate samplers (non-viable and viable), incubator, sterilizers (steam and dry heat), biological indicator and chemical integrator and pH meter)
Pharmacy technicians
- Demonstrate donning and doffing, hand hygiene, aseptic manipulations and sanitization techniques
- Demonstrate the use of closed system transfer devices for hazardous drug compounding
- Prepare compounded sterile preparations in an ISO classified three-room cleanroom suite
- Solve adjustment calculations for drug form and certificate of analysis-related indications
- Solve overage calculations for processing, sterility and endotoxin testing
- Sketch a cleanroom suite for optimal workflow
- Complete a Master Formulation Record
- Explain how to perform the gloved fingertip and thumb sampling and a media-fill test
- Describe findings of a risk assessment, and then construct a corrective action and preventive action plan
- Describe findings following an inspection, and then construct a corrective action and preventive action plan
- Operate electromechanical equipment (e.g., balance, automated compounded device, particulate samplers (non-viable and viable), incubator, sterilizers (steam and dry heat), biological indicator and chemical integrator, pH meter, and more)
Testimonials
“Having recently taken a position in a hospital setting, I decided to build on my basic knowledge of sterile compounding. I chose LP3 Network again, in my opinion, they are recognized as experts in this facet of pharmacy, and justifiably so. I highly recommend this program to any person who has the desire to excel in their compounding skills, because you can only be one of the best, if you learn from the best.”
“The course provided me with valuable, relevant and applicable information on sterile compounding in the comfort of a beautiful practice site. The combination of highly knowledgeable instructors, didactic written materials, hands-on-practical experience in a state-of–the-art lab that reflects a matrix of sterility with sparkling glass and mirrors throughout, provided me with one of the finest programs in my 30+ year career. I came away with having a proactive, rather than reactive approach to the preparation of compounded medications. My confidence has been 'compounded', no pun intended!
Pauline A. D’Chiutiis | Pharmacist
“What a wonderful program! The instructors use different types of learning throughout the entire program to grab the attention of every type of learner, through lecture, hands-on, and peer education. The practical component puts all the literature into perspective and the instructors teaching this course are absolutely A1!
Stephanie Armstrong | Pharmacy Assistant
FINANCIAL SUPPORT
This learning activity has received financial support from MEDISCA in the form of an educational grant.
COPYRIGHT
This CE Activity is Copyright © 2006-2026 LP3 Network.
Contributors
Jennifer Clare, BSc
Facilitator, LP3 Network
CE Program Developer, Inventory & Laboratory Logistics, LP3 Network
Neil Cohen, BSc
CE Program Developer, LP3 Network
Mark Filosi, BS Pharm, RPh
Facilitator, LP3 Network
President of Family Care Pharmacy and Beyond the Pill Wellness
Success Partner for Medisca Pharmacy Coaching Services
Michael Shafor, BSc, PharmD
LP3 Network - Program Developer and Facilitator
Medisca Network - Consultant
Osmotic International LLC – Owner and Consultant
Adjunct Professor, Nova Southeastern University
Facilitation Team
Jennifer Clare, BSc
Facilitator, LP3 Network
CE Program Developer, Inventory & Laboratory Logistics, LP3 Network
Roberto Conte, BS Pharm, RPh
Facilitator, LP3 Network
Manager, Sterile Compounding, McKesson Specialty Pharmacy (BC)
Mark Filosi, BS Pharm, RPh
Facilitator, LP3 Network
President of Family Care Pharmacy and Beyond the Pill Wellness
Success Partner for Medisca Pharmacy Coaching Services
Kenneth Latta, BS Pharm, RPh, FIACP, FACA
Facilitator, LP3 Network
Senior Associate, Gates Healthcare Associates
Consultant, Accreditation Commission for Health Care (ACHC/PCAB)
Vice President of Design and Regulatory Compliance for ProPharma Cleanrooms, Inc.
Consultant, North Carolina Board of Pharmacy
Senior Associate, Visante UK & Visante Inc.
William Perks, BSc Pharm, RPh, EMBA
Facilitator, LP3 Network
Manager of Pharmacy Manufacturing, Sunnybrook Health Sciences Centre
Christine Roussel, PharmD, BCOP
Facilitator, LP3 Network
Consultant, Medisca Network
Senior Executive Director, Pharmacy, Laboratory and Medical Research, Doylestown Hospital
Board Certified Oncology Pharmacist
Board Certified Sterile Compounding Pharmacist
Adjunct professor at the University of the Sciences in Philadelphia
Pennsylvania Board of Pharmacy, Vice Chair
Pennsylvania Medical Marijuana Advisory Board, Chair of Regulatory Sub-committee
Delegate, American Society of Health System Pharmacists (ASHP)
Past-President of the Pennsylvania Society of Health Systems Pharmacy
Board of Directors for International Society of Cannabis Pharmacists
Michael Shafor, BSc, PharmD
LP3 Network - Program Developer and FacilitatorMedisca Network - Consultant
Osmotic International LLC - Owner and Consultant
Adjunct Professor, Nova Southeastern University
Ken Speidel, BS Pharm, PharmD, RPh, FIACP, FACA
Facilitator, LP3 Network
Consultant, Medisca Network
Vice President, Compounding Compliance, Gates Healthcare Associates
Surveyor and Accreditation Expert, Accreditation Commission for Health Care (ACHC/PCAB)
Fellow, International Academy of Compounding Pharmacists (IACP)
Fellow, American College of Apothecaries (ACA)
Professor of Pharmacy Practice (retired), University of Findlay
Pharmacists & Pharmacy Technicians
Both the Sterile Compounding: Home Study Manual and Sterile Compounding: Hands-on Lab Training are accredited for Pharmacists and Pharmacy Technicians by the Accreditation Council for Pharmacy Education (ACPE) through CPE Consultants, LLC.
Total CPE Credits: 50 CPE Hours = 5.0 CEUs
| Sterile Compounding: Home Study Manual | Sterile Compounding: Hands-on Lab Training | |
| Type | Knowledge-based | Application-based |
| UAN | 0864-9999-25-044-H07-P/T | 0864-9999-24-051-L07-P/T |
| Credits | 30 CPE Hours = 3.0 CEUs | 20 CPE Hours = 2.0 CEUs |
| Release Date | July 1, 2025 | July 1, 2024 |
| Expiration Date | July 1, 2026 | July 1, 2027 |
Completion Requirements
Home Study
- Complete the home study learning assessment with a score of 80% and submit a completed home study evaluation.
Hands-On Lab Training
- Attend the lab training in full.
- Sign-off on your completed hands-on lab training performance assessment.
- Ensure your facilitator(s) have signed the performance assessment as well.
- Submit a completed hands-on lab training evaluation form online within 14 days of the training.
When the aforementioned steps have been completed and approved:
- Pharmacists and Pharmacy Technicians registered in the United States will obtain a statement of completed credits on their NABP e-Profile within 60 days.
- Pharmacists and Pharmacy Technicians registered elsewhere will receive a statement of completion by email within 60 days.
- All participants will receive an automated email from LP3 Network advising participants that they can download the LP3 Network certificate.
 | CPE Consultants, LLC is accredited by the Accreditation Council for Pharmacy Education as a provider of continuing pharmacy education. |
International participants should verify with their respective governing board for accreditation equivalency.
Please see below the list of available dates:
- Aventura, FL – February 27 - March 1, 2026 | Sterile Compounding: Certificate Program
- Vancouver, BC – March 6-8, 2026 | Sterile Compounding: Certificate Program
- Aventura, FL – April 10-12, 2026 | Sterile Compounding: Certificate Program
- Aventura, FL – May 15-17, 2026 | Sterile Compounding: Certificate Program
- Aventura, FL – June 12-14, 2026 | Sterile Compounding: Certificate Program
- Aventura, FL – August 14-16, 2026 | Sterile Compounding: Certificate Program
- Aventura, FL – September 11-13, 2026 | Sterile Compounding: Certificate Program
- Aventura, FL – October 16-18, 2026 | Sterile Compounding: Certificate Program
- Vancouver, BC – October 23-25, 2026 | Sterile Compounding: Certificate Program
- Aventura, FL – November 20-22, 2026 | Sterile Compounding: Certificate Program

 Facebook
Facebook X
X LinkedIn
LinkedIn Forward
Forward