Virtual Seminar – October 16-17, 2021
ESSENTIAL ELEMENTS OF HAZARDOUS DRUG COMPOUNDING

The live stream will take place according to Eastern Daylight Time (EDT).
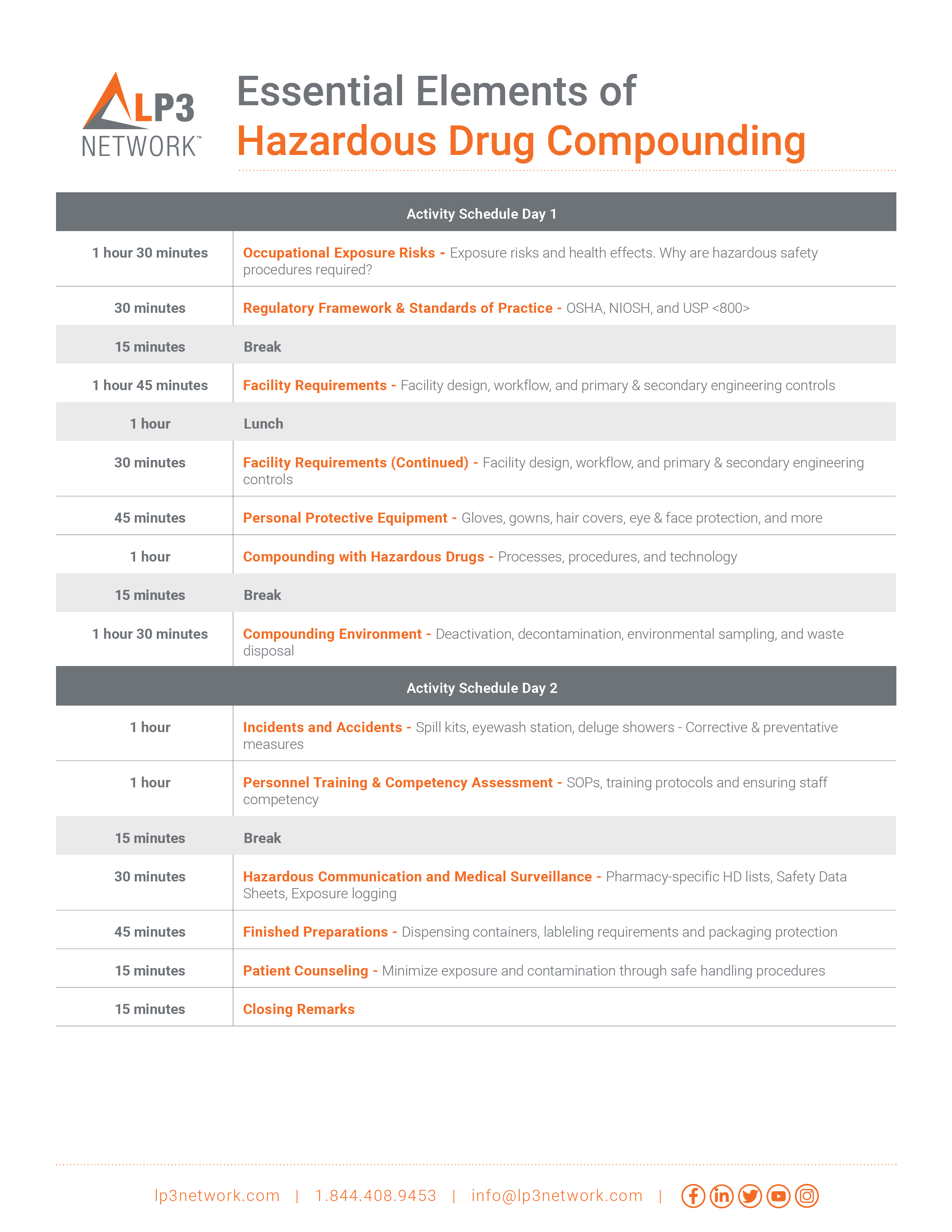
Day 1 - 9:00am to 6:00pm EDT
Day 2 - 9:00am to 1:00pm EDT
Course Format
1.5-day live virtual seminar (12 hours) that allows you to connect and interact with peers and the facilitator online, in real time, through open mic Q&A sessions, group discussions, polls, and online chats.
Course Description
Are you currently compounding with cyclosporine, estradiol, fluorouracil, phenytoin, progesterone, spironolactone, tacrolimus, testosterone, or any other drug found on the NIOSH List of Antineoplastic and Other Hazardous Drugs? If yes, then you want to comply with USP General Chapter <800> Hazardous Drugs – Handling in Healthcare Settings.
This application-based program is designed to take factual information presented in published USP, OSHA, NIOSH, and other healthcare guidelines and provide practical and applicable solutions to achieving compliance and safety within your practice. This program takes abstract concepts and makes them tangible and sensible. Participants will not just learn about the theory of facility requirements, they will develop their own facility blueprints and discuss and optimize them as a group. Participants will learn how to assess risk from personnel training & competency, to personal protective equipment (PPE), to drug handling, compounding processes, cleaning & deactivation, use of closed-system transfer devices, engineering controls, storage, and more. Participants will go through applicable scenarios and real-life situations that will shed light on exposure risk and how to correct it. The practical application and implementation of the requirements outlined in USP <800> can be challenging. This training provides you with the resources and insight for transitioning into compliance or maintaining compliance with USP <800>.

Concerned about USP <800> and the implications for you and your practice? Browse our FAQ for an idea of the topics we address in full during our Hazardous Drug Compounding seminar.
Intended audience
Pharmacists, pharmacy technicians, pharmacy managers, quality assurance officers, and compliance specialists looking for practical and applicable solutions to achieve compliance with USP General Chapter <800> Hazardous Drugs – Handling in Healthcare Settings.
Learning Objectives
Pharmacists
- Describe the hazardous compounding regulatory framework and standards of practice, including OSHA, NIOSH and USP <800>.
- Examine the risks of working with hazardous drugs in the pharmacy and healthcare continuum.
- Construct a pharmacy-specific hazardous drug list by assessing the occupational risks associated with hazardous drugs.
- Evaluate the critical strategies required to protect personnel, patients, and the environment when handling hazardous drugs in a healthcare setting.
- Assess hazard communication and training requirements.
- Contrast containment primary engineering controls (C-PECs) and their application.
- Interpret facility design plans for non-sterile and sterile hazardous drug compounding.
- Manage safe handling practices required for hazardous drug handling and compounding.
- Evaluate deactivation and decontamination procedures.
- Select personal protective equipment based on the NIOSH tiered approach.
- Demonstrate donning and doffing procedures.
- Examine the advantages of medical surveillance and environmental monitoring.
Pharmacy Technicians
- Describe the hazardous compounding regulatory framework and standards of practice, including OSHA, NIOSH and USP <800>.
- List the risks of working with hazardous drugs in the pharmacy and healthcare continuum.
- Construct a pharmacy-specific hazardous drug list by assessing the occupational risks associated with hazardous drugs.
- Outline strategies required to protect personnel, patients, and the environment when handling hazardous drugs in a healthcare setting.
- Recognize the importance of hazard communication and training requirements.
- Describe the different containment primary engineering controls (C-PECs) and their rationale for use.
- Reproduce facility design plans for non-sterile and sterile hazardous drug compounding.
- Outline safe handling practices required for hazardous drug handling and compounding.
- Describe deactivation and decontamination procedures.
- Select personal protective equipment based on the NIOSH tiered approach.
- Demonstrate donning and doffing procedures.
- List the advantages of medical surveillance and environmental monitoring.
Hardware/software requirements
We will be using Zoom for this Virtual Seminar. We recommend joining from a desktop or laptop computer - full system requirements can be found here. You can download the Zoom client here.
If you will be joining from a tablet or mobile device, check out the system requirements here. More information: Getting started on Android and iOS.
| Web browser |
| ||
Internet connection |
| ||
Misc |
| ||
Hardware | Minimum | Recommended | |
| Processor | Single-core 1Ghz or higher | Dual-core 2Ghz or higher (Intel i3/i5/i7 or AMD equivalent) | |
| RAM | N/A | 4 GB | |
Testimonials
"The instructor has a lot of experience and is very enthusiastic, making the course very enjoyable. I definitely recommend this seminar to my peers. It taught me the skills to develop my practice and I am already applying what I learned!”
Ana Gonzalez | Pharmacist
FINANCIAL SUPPORT
This learning activity has received financial support from MEDISCA Inc. in the form of an educational grant.
COPYRIGHT
This CE Activity is Copyright © 2016-2026 LP3 Network.
CHRISTINE ROUSSEL, PharmD, BCOP, BCSCP |  |
Dr. Christine Roussel is a Board Certified Oncology Pharmacist and Board Certified Sterile Compounding Pharmacist with a bachelor's in Toxicology and doctorate in Pharmacy from Philadelphia College of Pharmacy. Senior Executive Director of Pharmacy, Laboratory and Medical Research at Doylestown Hospital (Pennsylvania), Adjunct Assistant Professor at USciences, Facilitator for LP3 Network, and Consultant for MEDISCA Network. On top of her day job of overseeing the clinical and operational aspects of patient care, she provides over 100 hours of education to pharmacists, pharmacy technicians, physicians, and nurses each year.
Dr. Roussel focuses on occupational exposure to hazardous drugs, sterile/non-sterile compounding, clinical and legislative topics, such as medical cannabis, opioid stewardship, drug diversion prevention, pharmaceutical waste and contamination. She is a consultant for cleanroom facility design. She has authored articles on occupational exposure to hazardous drugs, as protecting healthcare providers’ DNA is her primary passion! Her most recent publication is: Meta-analysis of chromosomal aberrations as a biomarker of exposure in healthcare workers occupationally exposed to antineoplastic drugs. In 2018, she was nationally recognized as “40 Under 40 in Oncology”
She is active in many professional organizations, including as a Delegate for the American Society of Health Systems Pharmacy, President of the Pennsylvania Society of Health Systems Pharmacy, Board of Directors for International Society of Cannabis Pharmacists, the American Industrial Hygiene Association, and the Association for Compounding Pharmacists (ACP).
In addition to pursuing her goal of educating the world about the danger of carcinogens, she is also a dedicated mother, Cub Scout leader, and enjoys teaching her kids about engineering, often in the form of marshmallow propulsion devices and rocket building!
Editor
 ADAM BEACH, PhD ADAM BEACH, PhDLearning Technology Manager Disclosure: None |  |
Pharmacists & Pharmacy Technicians
The Live Event is accredited for Pharmacists and Pharmacy Technicians by the Accreditation Council for Pharmacy Education (ACPE) through CPE Consultants, LLC.
Total CPE Credits: 12 CPE Hours = 1.2 CEUs
| Type | Application-based |
| UAN | 0864-9999-21-055-L07-P/T |
| Credits | 12 CPE hours = 1.2 CEUs |
| Release Date | October 16, 2021 |
| Expiration Date | October 16, 2024 |
Completion Requirements
- Attend the live seminar in full (log in and logout times will be recorded).
- Submit a completed live event evaluation form online within 14 days.
When the aforementioned steps have been completed and approved:
- Pharmacists and Pharmacy Technicians registered in the United States will obtain a statement of completed credits on their NABP e-Profile within 60 days.
- Pharmacists and Pharmacy Technicians registered elsewhere will be able to download a statement of participation within 60 days.
- All participants will receive an automated email from LP3 Network advising participants that they can download the LP3 Network certificate.
 | CPE Consultants, LLC is accredited by the Accreditation Council for Pharmacy Education as a provider of continuing pharmacy education. |
International participants should verify with their respective governing board for accreditation equivalency.
CANCELLATION AND REFUND POLICY
In the event that LP3 Network cancels a course, LP3 Network's sole liability shall be course repayment. In no event shall LP3 Network be responsible for any costs related to travel and/or accommodation. A written request must be sent to LP3 Network Inc. requesting ‘Cancellation without Transfer’ to an alternate live activity event date. If cancellation occurs at greater than or equal to 31 days from live activity event date, then registrant will receive a 75% refund; 15-30 days a 50% refund; and less than or equal to 14 days no refund. There are no refunds, returns, or transfer requests upon purchase of a home study, digital work book, webinar, and eLearning modules. LP3 NETWORK INC. shall be excused from any delay caused by reason of any occurrence or contingency beyond its reasonable control (a “Force Majeure”), including but not limited to, acts of God, hurricane, earthquake, labour disputes, strikes, riots, war, and governmental requirements. The obligation to pay money to LP3 NETWORK INC. in a timely manner is absolute and shall not be subject to this Force Majeure provision. In such event, LP3 NETWORK INC. will not issue any refunds, only transfer to an alternate live activity event date.
TRANSFER POLICY
The transfer policy is only in effect if requested greater than or equal to 14 days prior to the original live activity date. Failure to submit the “Transfer Request” at least 14 days prior to the original live activity date will default to the “Cancellation and Refund Policy”. The transfer policy can only be applied one (1) time. Transfer to a new live activity date must be within the current calendar year. Failure to attend the new live activity will result in no refund and will no longer be transferable. Confirmation of the new activity date is subject to approval by LP3 Network based on the number of available seats within the selected live activity. There are no refunds, returns, or transfers upon purchase of the home study.

 Facebook
Facebook X
X LinkedIn
LinkedIn Forward
Forward